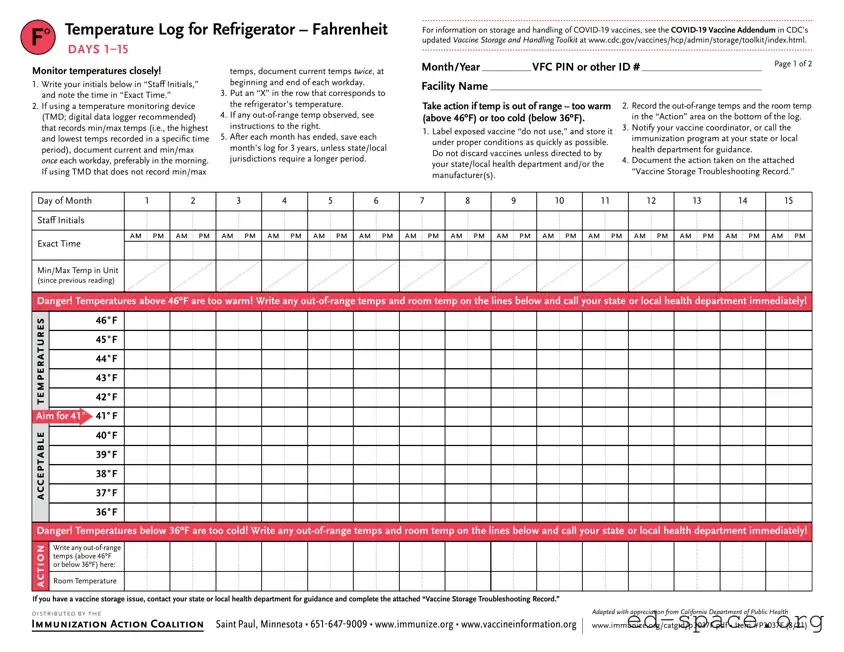
Vaccine Storage Troubleshooting Record (check one) |
◯ |
◯ |
◯ |
Refrigerator |
Freezer |
Ultra-Cold Freezer |
Use this form to document any unacceptable vaccine storage event, such as exposure of refrigerated vaccines to temperatures that are outside the manufacturers' recommended storage ranges.
Date & Time of Event |
Storage Unit Temperature |
Room Temperature |
Person Completing Report |
|
If multiple, related events occurred, |
at the time the problem was discovered |
at the time the problem was discovered |
|
|
see Description of Event below. |
|
|
|
|
|
|
|
|
|
|
|
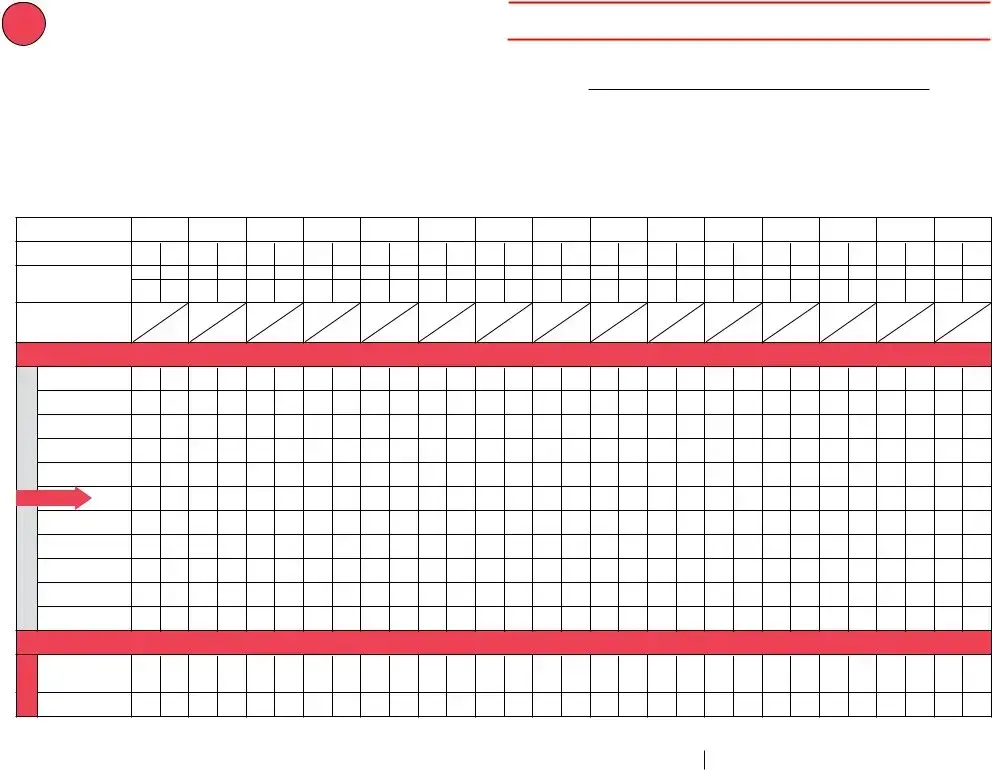
Date: (see below) |
Temp when discovered: |
45º F |
Temp when discovered: 77º F |
Name: Natalie Nurse |
|
|
|
|
|
|
|
Time: (see below) |
Minimum temp: 38º F |
Maximum temp: 53º F |
Comment (optional):temp is approx. |
Title: VFC Coordinator |
Date: 6/29/21 |
Description of Event (If multiple, related events occurred, list each date, time, and length of time out of storage.)
•General description (i.e., what happened?)
•Estimated length of time between event and last documented reading of storage temperature in acceptable range (2o to 8oC [36o to 46oF] for refrigerator; -50o to -15oC [-58º to 5ºF] for freezer; -80o to -60oC [-112º to -76ºF] for ultra-cold freezer (Pfizer COVID-19 vaccine only)
• Inventory of affected vaccines, including (1) lot #s and (2) whether purchased with public (for example, VFC) or private funds (Use separate sheet if needed, but maintain the inventory with this troubleshooting record.)
•At the time of the event, what else was in the storage unit? For example, were there water bottles in the refrigerator and/or frozen coolant packs in the freezer?
•Prior to this event, have there been any storage problems with this unit and/or with the affected vaccine?
•Include any other information you feel might be relevant to understanding the event.
At 8 am on Tuesday (6/29/21) morning when clinic opened, identified 4 temperature excursions over the weekend in refrigerator with readings as high as 54°, 50°, 49° & 53°F in primary vaccine storage unit #1. Recordings taken every 15 min on calibrated digital data logger overnight. Data logger probe in glycol located in middle of refrigerator with vaccines.
Total time out of range: approximately 3 hrs — maximum temp 53°F (see attached document of continuous temp readings)
Inventory of vaccines: see attached
Water bottles in refrigerator door. No vaccine stored in freezer. No problems with storage unit prior to Saturday night. Thunderstorms in area over weekend may have affected power.
Action Taken (Document thoroughly. This information is critical to determining whether the vaccine might still be viable!)
•When were the affected vaccines placed in proper storage conditions? (Note: Do not discard the vaccine. Store exposed vaccine in proper conditions and label it “do not use” until after you can discuss with your state/ local health department and/or the manufacturer[s].)
•Who was contacted regarding the incident? (For example, supervisor, state/local health department, manufacturer—list all.)
•IMPORTANT: What did you do to prevent a similar problem from occurring in the future?
Vaccines currently stored appropriately at 41ºF. Refrigerator and vaccines labeled "Do Not Use."
My State Immunization Program contacted at 8:30 am. Spoke with Victor Vaccine. Provided Victor with details of event and list of vaccines. Vaccine to remain quarantined until we hear back from Victor.
Called electric company and confirmed 2 short power outages during weekend. Checked refrigerator seals called refrigerator maintenance company to replace seals.
Checked plug on unit placed tape over plug to prevent inadvertent dislodging. Plan to purchase plug guard.
Plan to follow up with Immunization Program on data loggers with alarms that could be sent to coordinator and back-up phones.
Results
• What happened to the vaccine? Was it able to be used? If not, was it returned to the distributor? (Note: For public-purchase vaccine, follow your state/local health department instructions for vaccine disposition.)
Late on Monday, I talked with Victor regarding continued use of vaccine. Victor had checked with manufacturers which confirmed that vaccine is acceptable for use. He told me that vaccine could therefore be removed from quarantine. I discussed the entire situation with Susie Supervisor and Dr. Director (clinic medical director) who agreed that we could put vaccine back in use.