What is the Florida 3170 form used for?
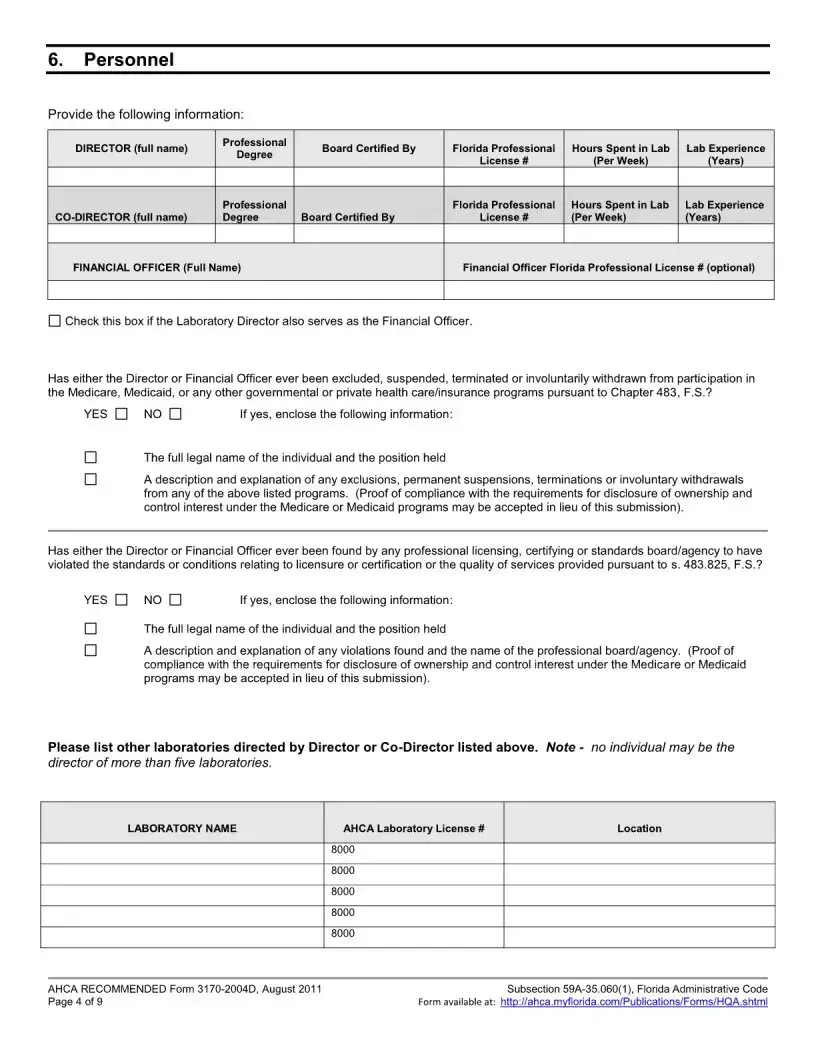
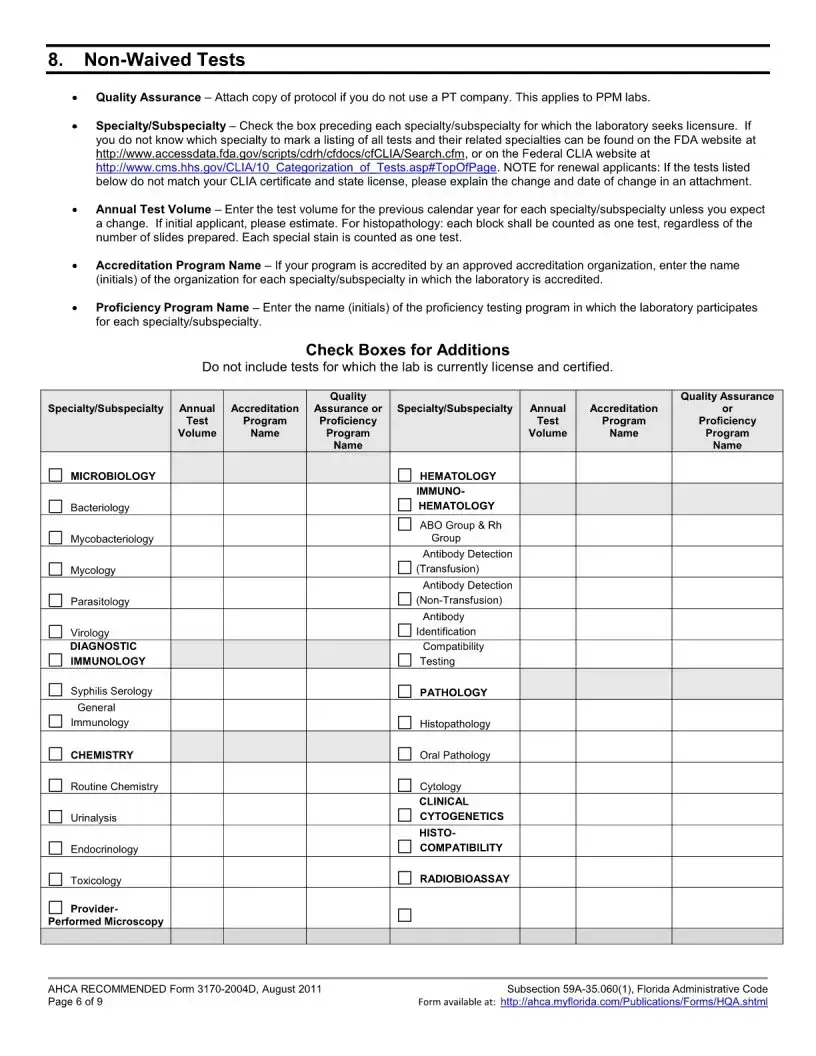
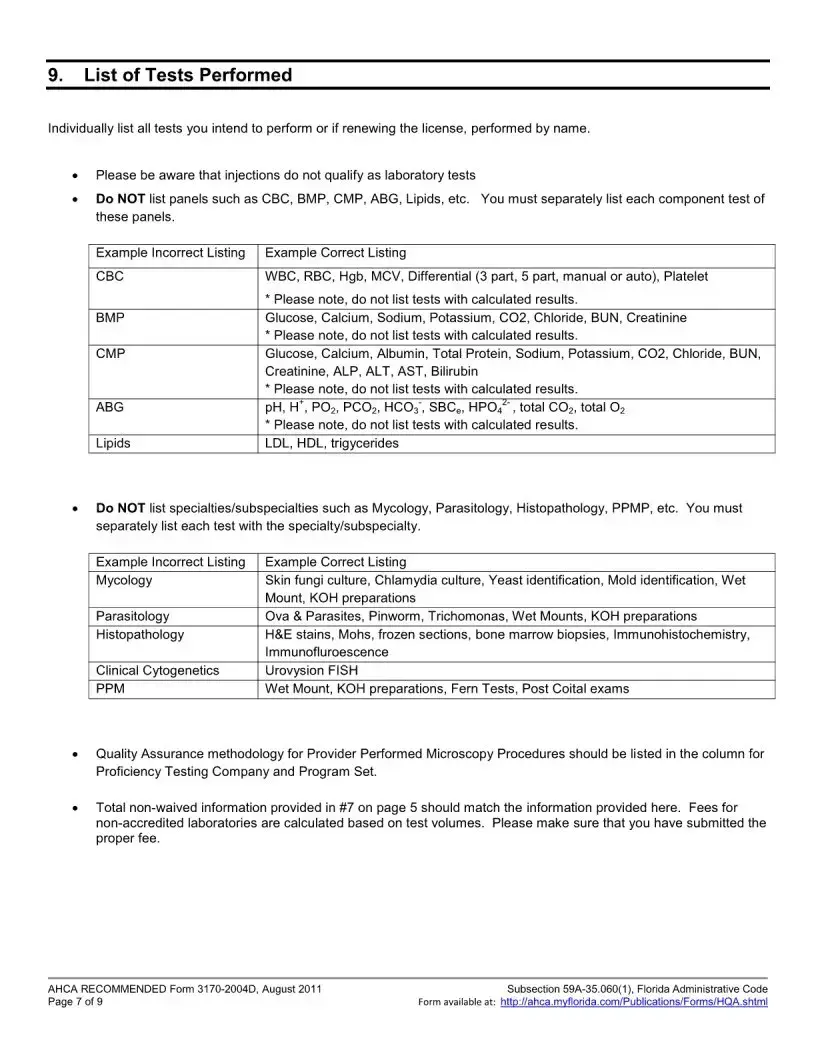
The Florida 3170 form is an application for health care licensing specifically for clinical laboratories that are non-waived. It is used when a laboratory seeks to add a specialty or subspecialty, or change its specialty outside of the regular licensure renewal period. This form ensures that laboratories comply with relevant Florida statutes and administrative codes regarding health care operations.
What documents must be submitted with the Florida 3170 form?
Applicants must include several attachments with the Florida 3170 form. These include a completed federal CMS 116 form, a copy of the medical or professional license, evidence of the director's qualifications, and proof of Level 2 background screening for both the Lab Director and Financial Officer. Additionally, an Affidavit of Compliance with Background Screening Requirements must be enclosed. All required documents must be submitted together to avoid withdrawal of the application.
What are the fees associated with the Florida 3170 form?
The application requires a licensure fee, which is outlined in Section 2 of the form. This fee is nonrefundable, and applicants must ensure that they submit payment in the form of a check or money order made out to the Agency for Health Care Administration. Starter checks and temporary checks are not accepted. Applicants may also incur late fees if the application is submitted less than 60 days before the expiration of the current license.
How long does it take to process the Florida 3170 form?
The processing time for the Florida 3170 form can vary. However, it is essential to submit the application at least 60 days prior to the expiration of the current license to avoid any delays or late fees. If the application is incomplete or if required documents are not submitted within 21 days of an omission notice, the application will be withdrawn from review. Timely submission of all required materials is crucial for efficient processing.
Where should the completed Florida 3170 form be sent?
The completed Florida 3170 form, along with all required attachments and fees, should be sent to the Agency for Health Care Administration, Lab Unit, at 2727 Mahan Drive, Mail Stop 32, Tallahassee, FL 32308. It is recommended to organize the documents neatly, placing checks and fingerprint cards on top of the application and paperclipping everything together to facilitate electronic storage.