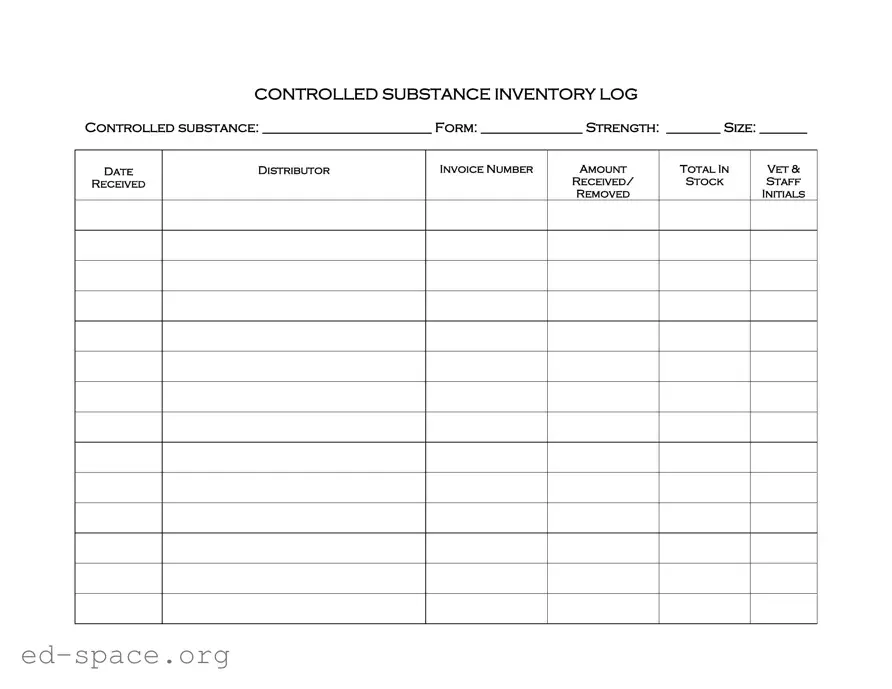
What is the purpose of the Controlled Drug Log form?
The Controlled Drug Log form is designed to track the inventory and distribution of controlled substances. This form helps ensure that all transactions involving these drugs are documented accurately, which is crucial for compliance with regulations and for maintaining proper records within a veterinary or medical setting.
What information is required on the form?
Each Controlled Drug Log form requires specific details. You will need to fill in the name of the controlled substance, the form it comes in (such as tablet, liquid, etc.), its strength, and size. Additionally, you must provide the date of receipt, the distributor's name, the invoice number, the amount received, and the total stock. Initials of the staff who received or removed the substance must also be recorded.
How often should the Controlled Drug Log be updated?
It is essential to update the Controlled Drug Log every time a new supply of a controlled substance is received or when any amount is removed from stock. Keeping the log current ensures accurate tracking and helps prevent discrepancies in inventory.
Who is responsible for maintaining the Controlled Drug Log?
The responsibility for maintaining the Controlled Drug Log typically falls on designated staff members, such as veterinarians, pharmacists, or veterinary technicians. These individuals must ensure that the log is accurately filled out and kept up to date to comply with legal requirements.
What should be done if there is an error in the log?
If an error is found in the Controlled Drug Log, it is important to correct it immediately. Draw a single line through the incorrect entry and write the correct information next to it. Initial the correction to indicate that it has been verified. Avoid using white-out or erasing entries, as this can lead to compliance issues.
How long should the Controlled Drug Log be retained?
The Controlled Drug Log should be retained for a minimum of five years. This duration may vary depending on specific state regulations or facility policies. Always check local laws to ensure compliance with retention requirements.
What are the consequences of not maintaining a proper Controlled Drug Log?
Failing to maintain a proper Controlled Drug Log can lead to serious consequences, including legal penalties, loss of licenses, and fines. Inaccurate records can also result in improper medication management, which can jeopardize patient safety.
Can the Controlled Drug Log be kept electronically?
Yes, the Controlled Drug Log can be maintained electronically as long as the system used meets regulatory requirements. Electronic logs should ensure data integrity, security, and easy access for audits. It is essential to have backup systems in place to prevent data loss.
Is training required for staff who handle the Controlled Drug Log?
Yes, training is highly recommended for all staff members who handle the Controlled Drug Log. Proper training ensures that everyone understands the importance of accurate record-keeping and complies with relevant laws and regulations. Regular refreshers can help maintain awareness and knowledge of best practices.