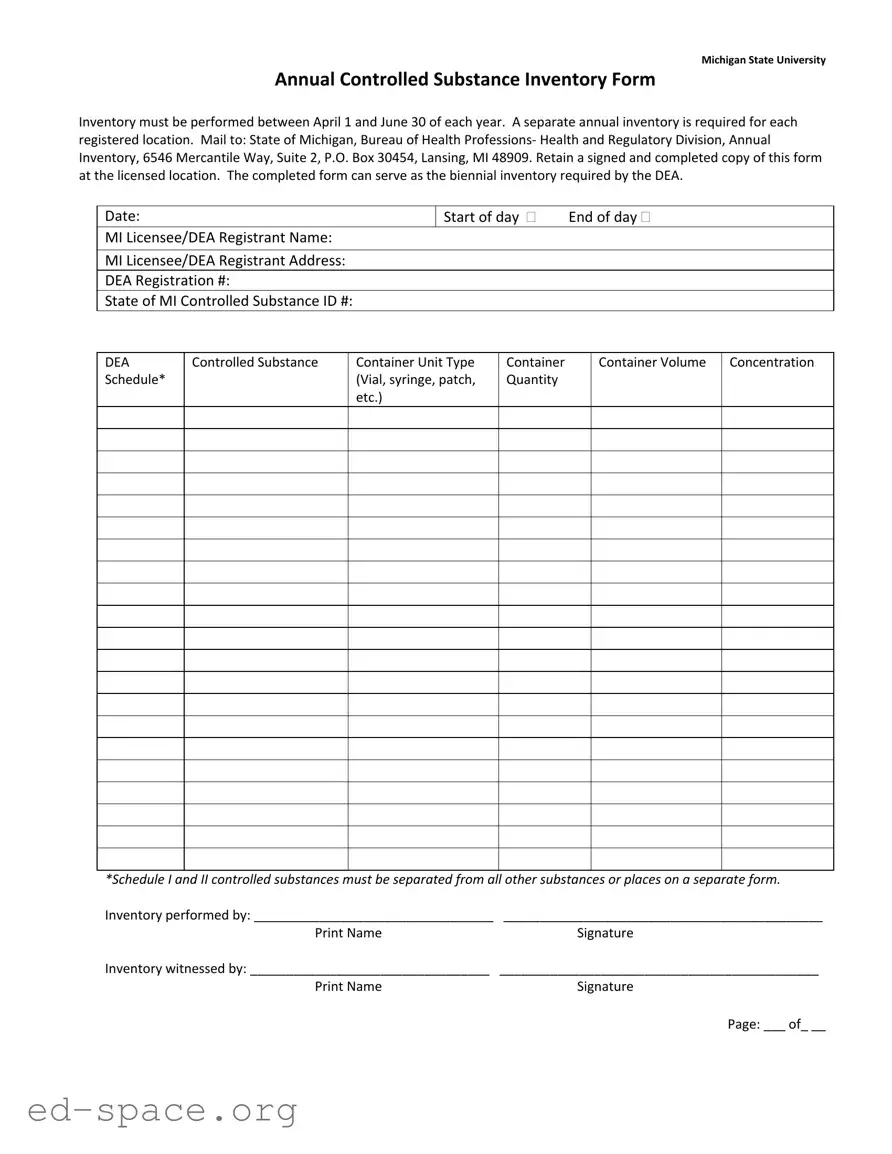
What is the purpose of the Controlled Substance Inventory Michigan form?
The Controlled Substance Inventory Michigan form is used to document the inventory of controlled substances at registered locations. It helps ensure compliance with state regulations and can also fulfill the biennial inventory requirement set by the DEA.
When should the inventory be performed?
The inventory must be conducted annually between April 1 and June 30. This time frame is set to maintain consistency and compliance with state regulations.
Do I need to submit a separate form for each location?
Yes, a separate annual inventory form is required for each registered location. This ensures that each facility's controlled substances are accurately accounted for and reported.
Where do I send the completed form?
Mail the completed form to the State of Michigan, Bureau of Health Professions‐ Health and Regulatory Division, Annual Inventory, 6546 Mercantile Way, Suite 2, P.O. Box 30454, Lansing, MI 48909.
What should I do with a signed copy of the form?
It is important to retain a signed and completed copy of the form at the licensed location. This copy serves as proof of compliance and can be referenced in future audits or inspections.
What information is required on the form?
The form requires details such as the date of the inventory, the name and address of the licensee or DEA registrant, DEA registration number, state-controlled substance ID number, and specifics about the controlled substances being inventoried, including type, quantity, and concentration.
How should Schedule I and II substances be handled on the form?
Schedule I and II controlled substances must be separated from all other substances. They should either be documented on a separate form or clearly identified to ensure compliance with regulations.
Who should perform and witness the inventory?
The inventory should be performed by a qualified individual, whose name and signature must be recorded on the form. Additionally, a witness is required to sign and print their name, confirming that the inventory was conducted accurately.